EU REACH: What Cosmetics Companies Need to Know
The European Union has one of the most protective policies against many chemicals commonly found in cosmetics. Even though these restrictions are part of European legislation, they do still apply to U.S. cosmetics manufacturers in many cases.
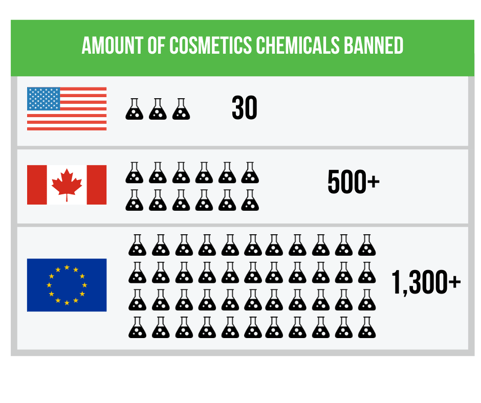
US - EU Differences in Banned Chemicals
There are major differences in what the United States allows in cosmetics compared to the European Union. In fact, the EU has banned or limited more than 1,300 chemicals for use in cosmetics, whereas the United States has only prohibited or restricted about thirty. That is 118 times more substances restricted through the EU Cosmetic Directive than that of the US Food and Drug Administration.
According to REACH policies, the restrictions apply to you if:
- You use ingredients that were purchased from a supplier located in the EU ( this is called being a downstream user)
- You are placing your cosmetics on the EU market
- You are in the EU and you are importing any cosmetics or ingredients that contain certain chemicals
- You are exporting chemicals, ingredients, or finished cosmetic products to the EU
How Does the EU REACH Regulate Cosmetics?
In the EU, the cosmetics industry and their products (raw materials or finished) are impacted by the EU Cosmetic Directive (76/768/EEC), which banned the initial 1,100 chemicals from cosmetics, the New EU Cosmetic Products Regulation (EC) No 1223/2009 and the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Regulation (EC) No 1907/2006.
REACH stands for Registration, Evaluation, Authorization, and restriction of CHemicals in the European Union. REACH was adopted to ensure that human health and the environment have a high level of protection. REACH also helps to combat the circulation of chemicals on the internal market and helps to promote innovating alternatives to animal testing and hazardous chemicals. Under REACH, it is the responsibility of the downstream user, the personal care or cosmetic company to comply with the regulation given they are importing substance of very high concern (SVHC) over 1 ton per year.
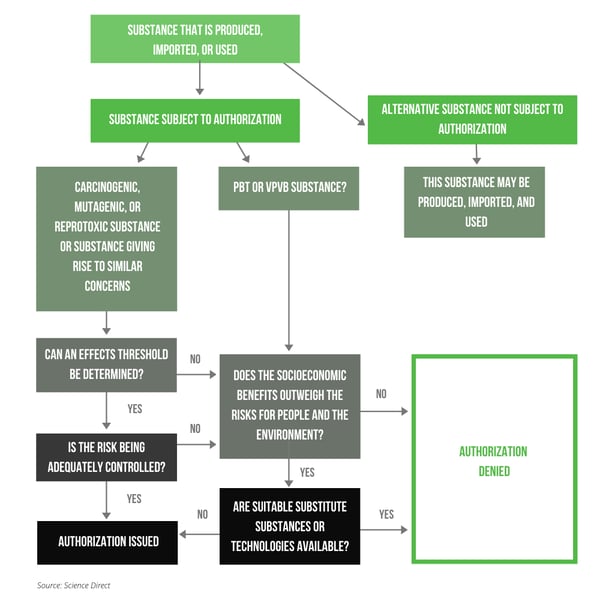
The EU accepts applications for authorization on a case-by-case basis. They use the following model when making decisions to grant authorization, which can be stripped upon restriction changes or if suitable substitutes become available.
How to Comply with the EU Cosmetic Directive
REACH reporting puts the burden of identification of SVHC’s within the supply chain on the producer or retailer. This can be complicated for the end-producer or retailer who does not have information on the material makeup from companies higher up in the supply chain.
At Source Intelligence, we are able to accept a large variety of chemical and material documents, which we can then run against the continually growing list of SVHC’s. Our user-friendly platform streamlines communications between you and your suppliers to provide greater transparency in your supply chain. We gather the information you need from your suppliers which you can then access in real-time reports on our platform’s dashboard.
To learn more about our REACH compliance program, click the link below!



